Choose the Most Stable Conformation of Trans-1 4-diethylcyclohexane
Draw the two chair conformers of each compounds and indicate which conformer is more stable. C methyl group-axial isopropyl group-axial.

Solved Choose The Most Stable Chair Conformer Of Chegg Com
A 1 B 2 C 3 D 4.
. In the most stable conformation of trans-14-dimethylcyclohexane what positions do the methyl groups. In the lefthand structure the two blue methyl groups are both axial on opposite faces of the ring. Cis and trans formspossible isomers of cis- and trans-14-dimethylcyclohexane.
14-diaxial conformation is least stable as the steric interactions are maximum. A methyl group-axial isopropyl group-equatorial. Equatorial position When a substitutent is present at axial position the conformer will be less stable because it has 1 3.
The most stable conformational isomer of trans-1-ethyl-2-methylcyclohexane will be. Consider the possible isomers of cis- and trans-14-dimethylcyclohexane. You can draw two flipped cyclohexane chairs.
When choosing the most stable conformation we look at both cis- and trans- isomers separately. Which of the following is the most stable conformation of trans-1-ethyl-3. If one methyl group is in the lower-energy equatorial position then the cis compound with both methyl groups on the same side of the ring can be made only by placing the second methyl group in the higher.
Therefore the total energy is 18 18 36 kcalmol. A cis-1-ethyl-3-methylcyclohexane b trans-1-ethyl-2-isopropylcyclohexane c trans-1-ethyl-2-methylcyclohexane d trans-1-ethyl-3-methylcyclohexane e cis-1-ethyl-3. Disubstituted cyclohexanes retain their cis- or trans- conformations even after a ring flip.
There is only one chair conformation of cis-14-dimethylcyclohexane. This means there are two possible forms for each cis-trans isomer. A cyclopropane B cyclopentane C cyclohexane D cycloheptane.
C cis-13-diethylcyclohexane D cis-14-diethylcyclohexane E trans-13-diethylcyclohexane 4 SHORT ANSWER. Each side of the ring is effectively an axial methyl cyclohexane. Which of the following Newman projections represents the most stable conformation of 23-dimethylbutane.
Predict the relative rates for the reaction of butadiene and a. And maleic acid 2. 28Draw the most stable conformation of trans - 1 - tert - butyl - 3 - methylcyclohexane.
Other articles where trans-14-dimethylcyclohexane is discussed. Write the word or phrase that best completes each statement or answers the question. The structure of cis-14-dimethylcyclohexane is.
View Test Prep - chem from BIO 321 at Hogwarts School of Witchcraft Wizardry. The more stable chair conformation of trans-12-dimethylcyclohexane has the two methyl groups in the equatorial position. Contrary to the case of methylcyclohexane which has no interactions in the chair conformation having an equatorial methyl group the diequatorial conformer of trans-12-dimethylcyclohexane has a gauche butane interaction red carbon.
Other articles where cis-14-dimethylcyclohexane is discussed. Lets look at cis- isomers first. The correct option is C In chair conformation of cyclohexane we have two position in the conformer.
Which of the following cycloalkanes has the least ring strain. What product is expected from the reaction of fumaric acid trans-2-butenedioic acid with 13-butadiene. The two conformations are identical.
What is the difference in energy between the two chair conformations of trans-14-dimethylcyclohexane. Which of the following has two equatorial alkyl substituents in its most stable conformation. You can also draw two flipped boat conformations.
Identify the compound where the groups are axial and equatorial. Cis and trans forms. In the most stable conformation of trans-1-isopropyl-3-methylcyclohexane what positions do the methyl and isopropyl groups occupy.
A 11-dimethylcyclohexane B cis-12-dimethylcyclohexane C cis-13-diethylcyclohexane D cis-14-diethylcyclohexane E trans-13-diethylcyclohexane. The two conformations are different but the one on the right is more stable. 14-Diethylcyclohexane C10H20 CID 14910 - structure chemical names physical and chemical properties classification patents literature biological activities.
14-diequatorial conformation is most stable as the steric interactions are minimum. D methyl group equatorial isopropyl group-axial. In each of the boxes below draw in methyl Me groups in the appropriate positions.
B methyl group-equatorial isopropyl group-equatorial. B trans-14-Dimethylcyclohexane shown below also exists in two different chair conformations one of which is 36 kcalmol more stable than the other. The stable conformers of trans-14-dimethyl cyclohexane is 1-equatorial-4-equatorial form.
If one methyl group is in the lower-energy equatorial position then the cis compound with both methyl groups on the same side of the ring can be made only by placing the second methyl group in the higher-energy axial position. Why is the trans-trans isomer of dibenzalacetone the most stable isomer. 4 points CH3 Least stable chair Most stable chair CH3G Ð36 kcalmol Question 3 is continued on the next page.
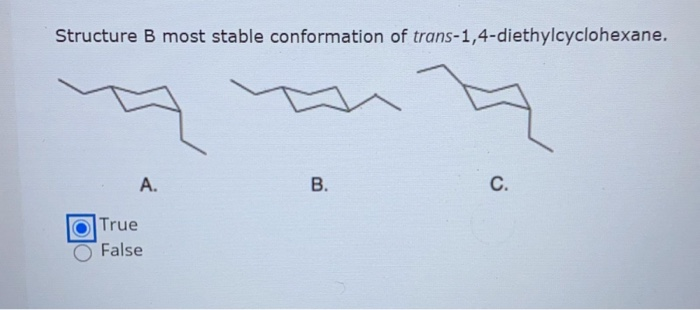
Solved Structure B Most Stable Conformation Of Chegg Com

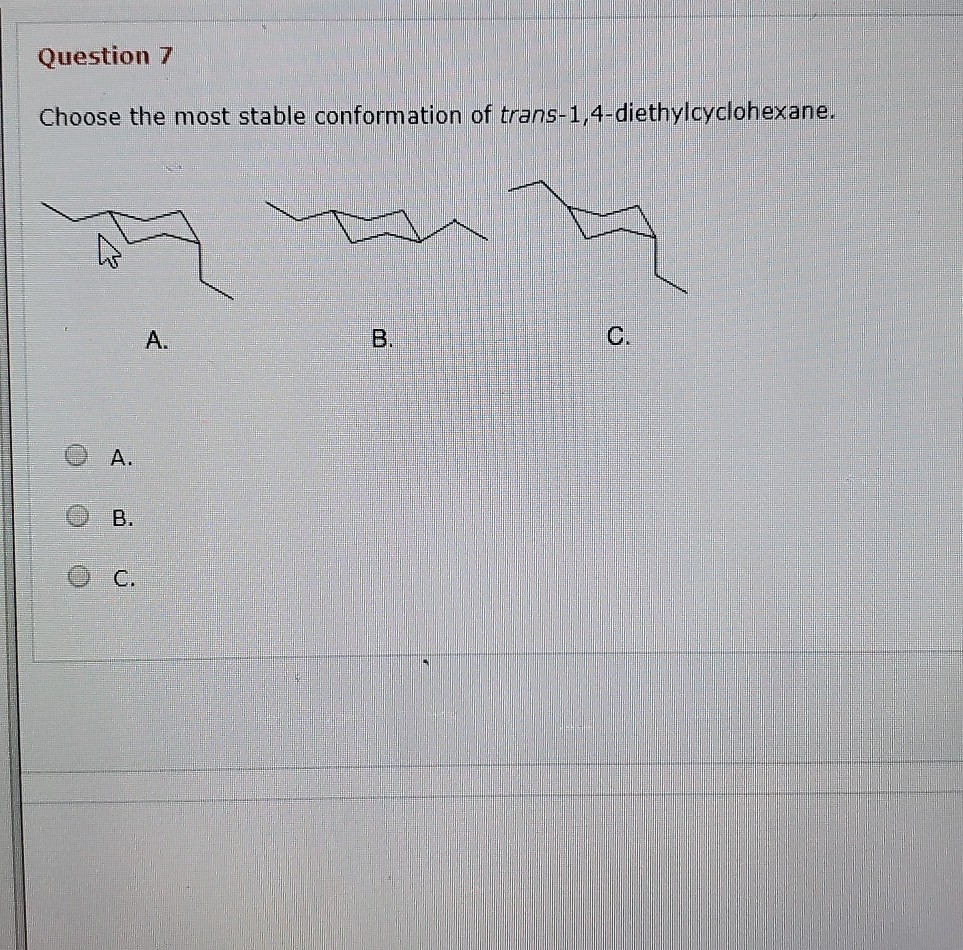
Solved Question 7 Choose The Most Stable Conformation Of Chegg Com
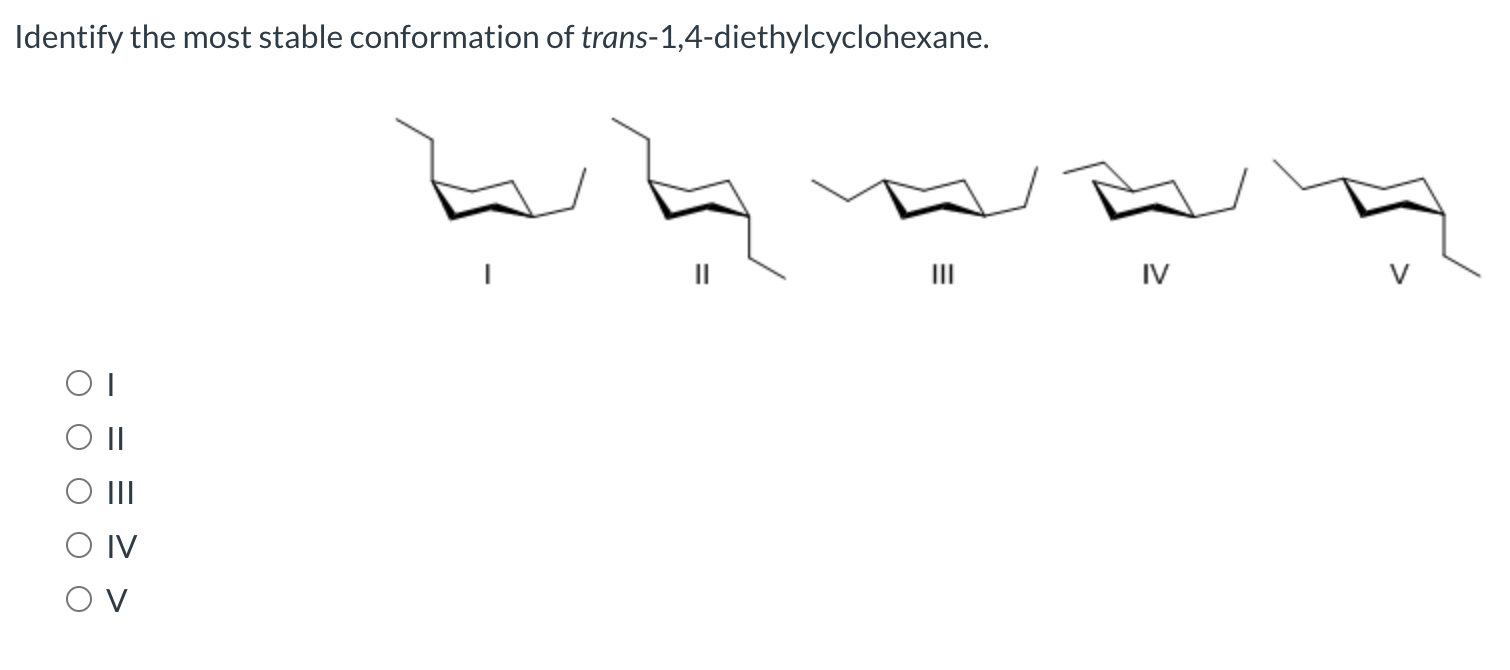
No comments for "Choose the Most Stable Conformation of Trans-1 4-diethylcyclohexane"
Post a Comment